Hydrogenation, the process of mixing hydrogen atoms to unsaturated oil, is a key chemical process in many industries like oil refining and is the base of turning crude to gasoline. In many similar industries, for example in production of plant oils like Crisco or margarine, this chemical process is essential. But, traditional ways of hydrogenation consume lots of energy and expensive metals. And so, it is not green by any means.
Researchers at Tufts have been working on a project to turn the hydrogenation process as green as possible. They have now smelt some success in their efforts as they could develop a selective hydrogenation catalyst, which is more inexpensive and eco-friendlier. Under the new process, single atoms of palladium, a commonly used hydrogenation catalyst, are scattered onto a copper base.
The researchers under Charles Sykes, associate professor in the School of Arts and Sciences, successfully managed to do the hydrogenation process in a greener way. The catalyst they used is less expensive and the process is green, claim the researchers. It will significantly bring many economical and environmental benefits for the industries.
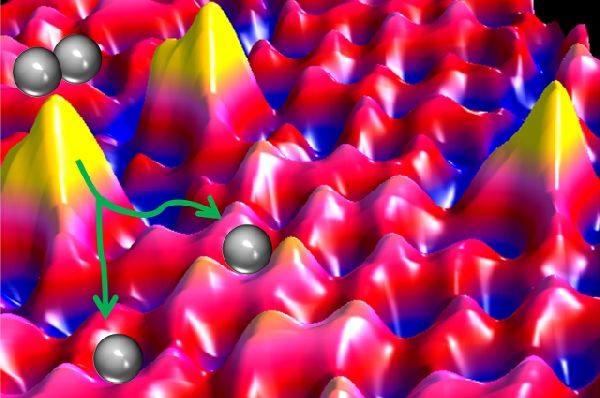
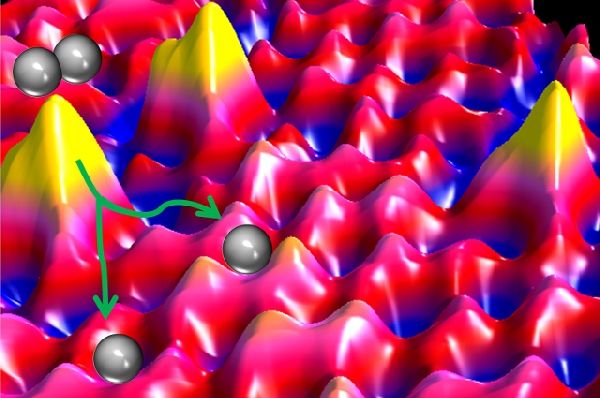
To test the green way of hydrogenation process, the Tufts scientists heated some amounts of palladium to nearly 1,000 degrees Celsius. At that heat, the metal dissolved like a gas and discharged single palladium atoms, which were embedded into a copper metal surface, placed around three inches away.
The resultant single palladium atoms in the process are less than half a nanometer wide. In conventional hydrogenation process, palladium required is 5 to 10 nanometers wide. It means that great amount of the expensive palladium is required for the process. But the outcome is same under both the processes, however. The Tufts’ research results were outed in the journal Science.
Via: Tufts