Hydrogen could easily be called the fuel of the future. It is a storehouse of extreme nuclear energy. Furthermore, even as a normal gas, it is highly combustible, which makes it an excellent source of fuel. However, because of this very combustibility, it is easy for the hydrogen reaction to go out of hand. Therefore, right now, we are not using it as extensively as we should. Nevertheless, there are many ways of utilizing this energy by limiting its power. Making a fuel cell out of it is one of the options. It isn’t that difficult. So read on if you want to know how to build a hydrogen fuel cell at home.
What is a fuel cell anyway?
 A fuel cell is a kind of device that converts hydrogen, methane or gasoline into electricity. It reduces the hard task that human beings used to do in the early ages. They are basically used in places like spaceships, where there is a requirement for effective electricity. Well, you can make this cell in your own kitchen in about 10 minutes and exhibit how oxygen and hydrogen can produce electricity jointly.
A fuel cell is a kind of device that converts hydrogen, methane or gasoline into electricity. It reduces the hard task that human beings used to do in the early ages. They are basically used in places like spaceships, where there is a requirement for effective electricity. Well, you can make this cell in your own kitchen in about 10 minutes and exhibit how oxygen and hydrogen can produce electricity jointly.
Difficulty Level:
Moderate
Time required:
10-15 minutes approx
Resources required:
1. A wire made of either pure platinum or nickel coated with platinum.
2. A stick made of wood or a wooden popsicle stick.
3. A battery clip of 9 volts.
4. A transparent sticky tape.
5. A glass full of water.
6. A voltmeter.
Steps to build a hydrogen fuel cell:
1. Cut the wire into long pieces measuring six inches each and twist each of the pieces into a small spring that has to be coiled. These will be the electrodes in our project. A nail or a coat hanger will do well as the coil formation.
2. Cut the leads of the clip of the battery and the insulation is to be stripped off at the cut ends. Then, the lead wires of the bare battery are to be winded onto the endings of the electrodes that are coated with platinum. To the electrodes, apart form the battery clip, two wires are going to be attached with. This will be used later on as a connection to the volt meter.
3. There is the popsicle stick to which the electrodes are attached safely while the stick is also securely attached to the glass containing water. This will make these electrodes hang down inside the water for their full length. Thus, the wire connections that are twisted should be kept out of the glass of water ensuring that the the electrodes coated with platinum are inside the same.
4. On the voltmeter, you will see two terminals: positive and negative. Get the red wire connected to the former while the black one to the latter. The meter must be reading 0 at this particular point. You may see a small quantity of volts, say 0.01.
5. With the above four steps your preparation is complete. Now, comes the point of operation. To put it on operation, it is required to get some hydrogen bubbles to take place to stick to one electrode and the oxygen bubbles to another. For this we need to follow these simple guidelines.
How to put your hydrogen fuel cell to work
 (i) The battery clip is touched by the 9-volt battery as they are not required to be clipped.
(i) The battery clip is touched by the 9-volt battery as they are not required to be clipped.
(ii) Now the clip is to be touched by the battery which will make the electrodes break into oxygen and hydrogen. This is what you call electrolysis. As the battery will be attached, you will be able to see the formation of bubbles at the point of the electrodes.
(iii) After the removal of the battery, there will be readings of zero volts on the volt meter if the platinum coated wire is not in usage.
(iv) The reaction of electrolysis gets reversed. The oxygen and hydrogen unites to create water and generate electricity. This happens due to the platinum that plays the role of catalyst which makes it possible.
(v) In the beginning, we get very little from this project of the fuel cell. As soon as the popping of the bubbles takes place, it does get dissolved in the water or the reaction uses it up. Then, the voltage starts dropping quickly and then slows down a bit.
(vi) As the voltage starts declining slowly, the main reason behind it is because of he gasses that are used up producing electricity.
6. Frequently asked questions:
(a) Where are fuel cells used?
Ans. They are used especially in the high-tech applications like the ones of spacecrafts where well-organized power source is required.
(b) Is it necessary to use a popsicle stick?
Ans. It isn’t very necessary to use the popsicle stick. You can use wood or a plastic stick as a substitute.
(c) Does this project ponder on electrolysis completely?
Ans. No. Apart from electrolysis, one more issue is a matter of concern out here i.e. recombining of oxygen and hydrogen to generate electricity.
7. Quick Tips:
(a) The electrons must be kept in metal wire as the current flows in the wire well than in water.
(b) Two aspects of this project are to be looked upon separately i.e. electrolysis and recombining of the gases to produce electricity.
(c) The oxygen and hydrogen can be bubbled from some other source to get electricity.
(d) Hydrogen and oxygen can be produced from the solar power and can be used later on at night in the fuel cell.
Some portable gadgets that operate using Hydrogen Fuel Cells
Okay, now you have your hydrogen fuel cell, but what do you do with it. Well, you can use it to provide energy to your gadgets. For example, here are some gadgets that work on hydrogen fuel cells:
1. Hydrogen Fuel Cell Powered Kettle
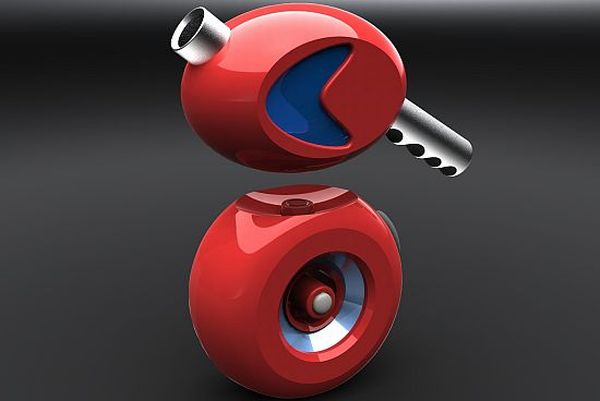
An industrial designer, Mathew Smith designed a wireless kettle which works on hydrogen fuel cells; it is compact, eco-friendly and can easily be transported wherever you go especially when camping out. The electricity is made by converting the hydrogen with the help of hydrogen fuel cells which are placed at the bottom half of the kettle in turn boiling the water in the top half. There is a ring of LEDs circulating the hydrogen cartridges which light up when the Kettle is switched on. It is easy to handle and has a big enough control button. The kettle is constructed for the future, to be used in the year 2100.
2. Powertrekk Portable charger

A gadget with which you can charge anything you need to anywhere. It was designed in order to provide the user with charging facilities which consists of a Li-ion battery pack, it can also be used as a fuel cell. Powertrekk consumes water in order to get the hydrogen which is then required to generate electricity. It is a convenient pocket-sized, eco-friendly gadget with hardly any weight. It is an efficient power generating source which works immediately, with the support of a PEM 4-cell unit, 1000mAH fuel cell placed within a waterproof case. Enjoy yourself with no worries of your cell-phone dying out, or your torch when you’re out in the dark without a plug point or any other source of electricity.
3. Horizon’s MiniPak fuel cell charger

The Horizons MiniPak provides a person with 1.5-2W of constant power with the help of an integrated micro-USB slot and multiple choice cable to charge other devices. One can use rechargeable hydrogen fuel cells with which energy can go up to 12W. A blend of the products highly produced PEM fuels and a metal hydride storage solution is used, which reserves the hydrogen as a dry, non toxic and non- pressurized material which is also non-harmful. An eco-friendly gadget in which hydrogen is stored and changed into a metal hydride with the help of a metallic sponge present in the fuel cell.
4. G2 Portable Fuel Cell Power Source

Charging your Cell phones, PDA’s, digital cameras and other gizmo’s without having to worry about a power source has become more efficient with the help of the G2 portable Fuel Cell Power Source. Designed by Angstrom, it comes with a USB Port. It consists of a 2.0W fuel cell system which is charged up due to a cluster of 8 V60 fuel cell modules. The only downside to having this product is that the hydrogen cells used need to refilling for that you need the station.
5. Fuel Cell Sticker

myFC, a Sweden based firm, has come up with a wireless, sustainable and portable fuel cell system which as small as a lithium battery. The fuel-cell-sticker, as the name speaks for itself is an adjustable 0.11 inch thick fuel cell weighing only 0.2 ounce. It contains a proton exchange membrane which enhances power density and efficiency. Furthermore, it produces 0.9W of power at 0.5W.



