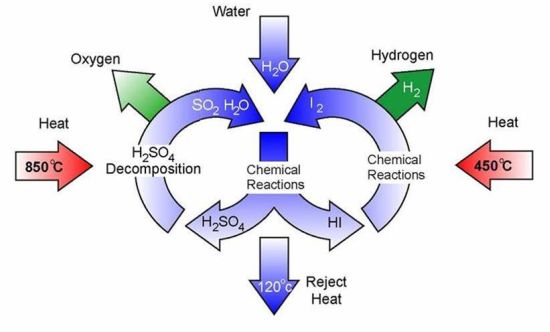
While much is being said of developing the next generation hydrogen fuelled hybrid cars and hydrogen fuelled batteries for mobile phones and a host of other electronic devices, non-availability of commercially viable methods of hydrogen production has acted as an impediment to manufacturers to mass produce hydrogen powered vehicles and gadgets. Taking a cue from the method of electron transfer and water oxidation during photosynthesis, a new proof-of-concept device has been developed by researchers at Pennsylvania State University that could split water and produce hydrogen.
The researchers have developed a catalyst that consists of iridium oxide molecules surrounded by orange-red dye molecules. They chose the orange-red dye as it had the capacity to absorb sunlight in the blue range that has maximum energy. The catalyst was then impregnated to a titanium dioxide electrode to act as the anode and a platinum electrode served as the cathode. The electrodes were then immersed in a salt solution and were separated from each other to avoid the component gases in the solution from recombining. Sunlight falling on the dye-sensitive anode would start the process of splitting water into its component gases.
However, this process of harvesting hydrogen by splitting water molecules using sunlight has not attained the efficiency level commensurate with commercialization of the technique for hydrogen production. Researchers are working on a number of approaches to improve the process that includes improving the efficiency of the dye, improving the catalyst and adjusting the general geometry of the system.